News, Stories and Product Launches
Uniplex (UK) Ltd
The latest advancement in flexible endoscope cleaning
Posted 1st May 2020
The cleaning of flexible endoscopes has always been considered difficult due to the inability to inspect the cleanliness inside the lumens of an endoscope¹, that is, until now. The latest advancements in technology have allowed the development of flexible inspection camera systems that can check the internal channels of an instrument for damage and debris, which could help in the reduction of infection rates.
There are two categories of Endoscopy-related infections². These are -1) Endogenous infections from the patient’s own microbial flora. 2) Exogenous infections caused by inadequately reprocessed equipment. Exogenous infections come from a pathogen entering the patient’s body from the external environment. One way in which this can occur is human error in the reprocessing of flexible endoscopes. It has always been notoriously difficult to ensure that the internal channels of a flexible endoscope are clean, free from debris, or articles that have no place to be in the channels such as stents from a previous procedure, all of which carry infection risks and potentially worse!
Cleaning Brushes
Previously, technicians have had to rely on the ability of the brush they’re using to perform the task and they put complete faith that if the brush is coming out clean, then the flexible endoscope must be clean. There are brush guidelines and best practices to try and ensure flexible endoscopes are cleaned sufficiently, but unless the correct size brush is being used and the most suitable technique applied, the brush may simply miss dirt in the channel. Such guidelines and recommendations can include “the size (length and diameter) and type of cleaning brush should appropriately match the size and type of the endoscope channel to ensure contact with channel walls, and access to all small/narrow lumens” ². It is known that some companies recommend, and hospital technicians use a one size fits all type brush, which can allow dirt and debris to slip pass the brush, which can lead to infections. After all, logic would suggest that any brush that can compress down a 1.2mm tube cannot possess much strength when facing a channel size four times larger. A brush needs to use the tips of the bristles to clear out any dirt/debris and therefore requires some strength behind them to achieve this. One size fits all channels are much flimsier than brushes that are created to fit the specific lumen size required.
“Alternative available technologies should be considered for the detection of residual proteins on the internal surfaces of flexible endoscopes following reprocessing” – Department of Health¹
Exogenous Infections
There are a vast range of infections that can occur from endoscopy procedures, including bacterial infections, infections from multidrug-resistant organisms, virus infections, Mycobacterial infections and more. It has yet to be reported but it is also theoretically possible to contract Creutzfeldt-Jakob disease (CJD) from poorly processed endoscopes². Multidrug-resistant Enterobacteriaceae have been found in flexible endoscopes such as duodenoscope. It has been suggested that this could be due to small cracks appearing over time, i.e. general wear and tear on the insertion tube as well as the instruments internal structures, damage that naturally and inevitably occurs as a result of normal wear or aging. The scope may function correctly, but the small cracks can act as a harbour for bacteria and these can expose a patient to an exogenous infection. Small cracks may have been considered unpreventable before now and unless the scope was sent to the manufacturer or a third-party service for repair. This could leave the user completely unaware of some of the issues as these would be completely invisible due to the construction of the scope.
The Department of Health
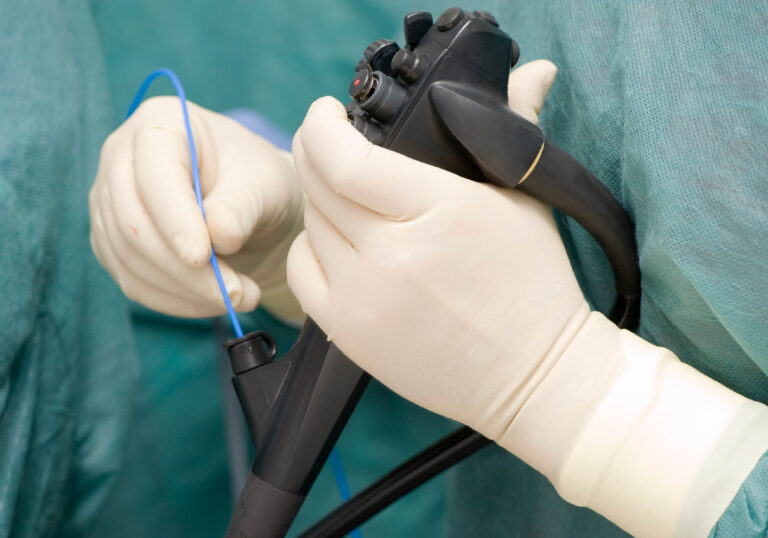
The Department of Health understands the difficulties when it comes to the cleaning of internal channels and lumens, but also knows the risks of essentially ‘blind cleaning’ endoscopes. This is why they have stated in their guidelines for cleaning flexible endoscopes that “alternative available technologies should be considered for the detection of residual proteins on the internal surfaces of flexible endoscopes following re-processing” ¹. Due to issues such as cracks and biofilms on the internal lumens, there has been a need identified for manufacturers to develop inspection cameras that can visibly check areas which were once considered impossible. One such camera is the FIS005* now readily available in the United Kingdom.
Flexible Inspection Systems
Cameras, not only in the medical industry, but in all sectors and markets are getting smaller, with higher clarity and better images. This can be seen in the ongoing advancement of smart-phone cameras. It’s this technology that has allowed the development of flexible inspection systems (FIS) that can be used to visually inspect inside flexible endoscopes. FIS cameras tend to work by having a long, thin, wire like camera that can pass down channels and lumens. Current FIS systems on the market can go down to a diameter as small as 2mm, allowing the camera to reach down tiny, hard to see and reach in areas.
Why the need for a camera that can look inside channels? FIS can visually check for damage and debris inside flexible endoscopes, this can help reduce the chances of infections in circumstances such as ineffective cleaning with the wrong size brush, unseen biofilms and checking for damage such as cracks and scratches on the internal walls that can harbour bacteria.
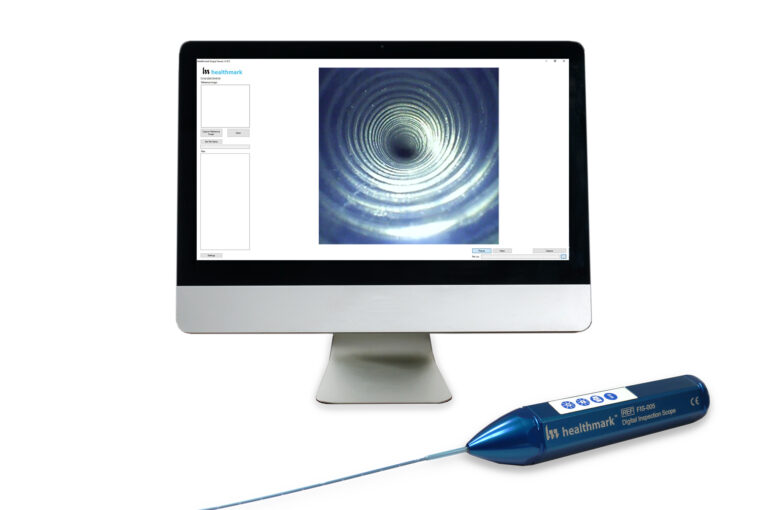
Documented Evidence
A flexible inspection scope [FIS] not only allows for live images of the inner surfaces of the various lumens and channels but can also be useful in the documentation of the cleaning and reprocessing journey of flexible endoscopes. The Society of Gastroenterology Nurses and Associates suggests that “quality assurance is essential to the continued safety and effectiveness of endoscope reprocessing”, and to help achieve this they suggest information about reprocessing activities is required3. FIS can assist this protocol by storing images and videos on an associated computer before, during and after the decontamination process. These images can then be reviewed and act as evidence if and when required.
Conclusions
It’s essential to follow the latest guidelines by governing bodies to ensure best practice. Following these guidelines suggests that a Flexible Inspection System should be considered as they could potentially reduce infection rates, thus giving a better patient outcome, whilst also saving money for the NHS
References
1. Department of health, ‘Health technical Memorandum 01-06: Decontamination of flexible endoscopes’, Management and decontamination of flexible endoscopes (HTM 01-06), <https://www.gov.uk/government/publications/management-and-decontamination-of-flexible-endoscopes> [accessed 03 March 2020]
2. Ulrike Beilenhoff et al, ‘Reprocessing of flexible endoscopes and endoscopic accessories used in gastrointestinal endoscopy: <ESGE – ESGEN Position Statement- updated 2018> [2018]
3.Society of Gastroenterology Nurses and Associates Inc, ‘Standards of infection prevention in reprocessing flexible gastrointestinal endoscopes’ <SGNA> [2016]
Cost-effective haemostat to achieve savings for theatres
In this current climate, in which public spending is coming under closer scrutiny, Uniplex believes that a review of expenditure could prove useful and revealing for…
Sunoptic’s brightest LED headlight
The innovators at Sunoptic Surgical have launched their brightest battery-powered LED headlight to date – the SSL-9500. The experts in surgical lighting…
Partnership helps surgeons heal wounds
In the middle of 2016 a strategic partnership was formed between Meril Life Sciences and Uniplex UK Ltd to introduce a premium brand of absorbable and…
Request A Quote